Regulatory Services
End-to-end support for market access.
GMP Services Tailored for You.
Export Services for Global Reach.
GMP Services
Export Services

Your Trusted Partner for
Regulatory & GMP Services
At RGInsight LLP, we provide integrated support to pharmaceutical and biologics companies seeking to expand into Regulated, Emerging, and Domestic (India) markets. Our approach combines regulatory, technical, and commercial expertise to ensure smooth and compliant market entry.
We assist clients with GMP compliance services, including manufacturing site assessments, gap identification, inspection readiness programs, and coordination with Qualified Persons (QPs) and local regulatory authorities. Whether preparing for EU-GMP, USFDA, or WHO inspections, our team ensures that your facility and documentation are aligned with the latest global standards.
For companies preparing to enter new markets, RGInsight offers complete support for Marketing Authorization Applications (MAA). This includes country-specific regulatory strategy, preparation and submission of dossiers in CTD/eCTD formats, query handling, and lifecycle management services such as variations, renewals, and MA transfers. We work closely with local agents and regulatory consultants in each region to ensure accurate, timely submissions and approvals.


In addition to regulatory support, we help our clients build a strong commercial presence by identifying and connecting them with trusted local distributors and marketing authorization holders (MAHs). Leveraging our well-established global network, we facilitate commercial alliances that are both compliant and strategically aligned, helping clients achieve sustainable growth in new markets.
Whether you are launching your first product internationally or expanding your existing footprint, RGInsight acts as your strategic partner—streamlining regulatory pathways and unlocking market potential across global geographies.
Global Regulatory Experience
















Europe
ASEAN
CIS
Africa
MENA
LATAM
GCC
Canada

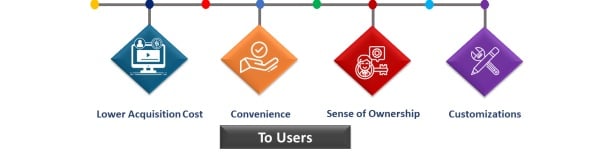
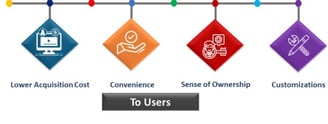
Comprehensive Regulatory Support
Expert services for regulatory, quality, and export needs in pharmaceuticals and biologics for businesses.
Global Network Access
Connect with trusted distributors and enhance international collaborations for seamless market entry and growth.


End-to-End Services
We provide complete regulatory support, ensuring faster and cost-effective access to global markets.
Our industry experts guide you through every step of the regulatory process for successful market entry.
Expert Guidance


Our Regulatory Service Models


For Specific Projects or one-time requirements
A Smarter Approach to Regulatory Submission Management
Managing regulatory affairs doesn’t have to be complex or costly. With our subscription-based regulatory support model, you gain access to a dedicated team of trained professionals—without the hassle of hiring, training, or managing an in-house department. Whether you're a startup scaling up or an established company aiming to streamline compliance, our fixed-cost model ensures clarity, control, and continuity.
Our Project-Based Regulatory Outsourcing Model is designed to provide end-to-end regulatory support tailored to specific projects or deliverables—offering maximum flexibility, cost-efficiency, and domain expertise. You can get access to a dedicated team of experts in CMC, clinical/non-clinical, eCTD publishing, regulatory strategy, and region-specific requirements for your small no. of projects.
Subscription Based
Fixed Monthly Cost. Predictable Budget.
Annual Product Registration Included.
Operational Savings Without Compromise.
Expert Regulatory Support—Always On.
No Hassle of Training—Skilled Professionals at Your Service.
No Recruitment Headaches—We’ve Got You Covered.
Project Based
Defined Scope, Timelines & Deliverables
No Long-Term Commitment
Access to Multi-Disciplinary Expertise
End-to-End Regulatory Execution
Scalable & Agile
Ideal For
SMEs with no or small in-house regulatory team
Companies entering new markets,
Clients requiring quick turnaround for urgent submissions
Partnering for dossier readiness pre-out-licensing

Get in Touch
Contact us for regulatory and export service inquiries today.


