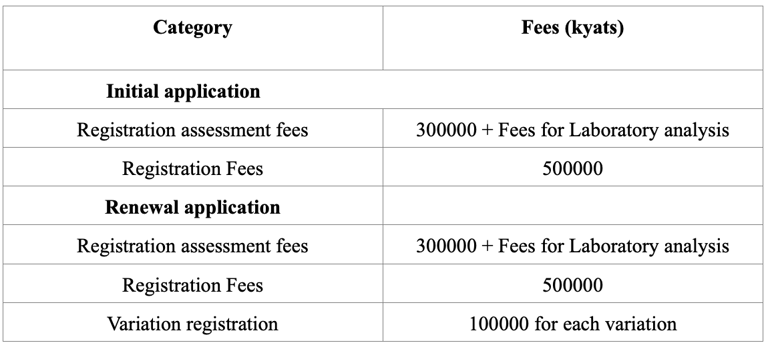
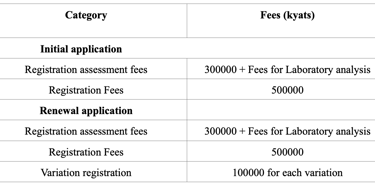
Registration Of Drug Product In Myanmar (2025)
Overview
In Myanmar, drug product registration is regulated by the Food and Drug Administration (FDA Myanmar) under the Ministry of Health. All pharmaceutical products intended for import, manufacture, or sale in Myanmar must be registered with FDA Myanmar to ensure safety, efficacy, and quality. The registration process requires submission of a dossier in the ASEAN Common Technical Dossier (ACTD) format, covering administrative, quality, non-clinical, and clinical data. The application must be submitted by a locally licensed company that is authorized to act as the Marketing Authorization Holder (MAH).
The regulatory authority performs both administrative and technical reviews. The technical assessment includes evaluation of pharmaceutical data (formulation, stability, and GMP compliance), as well as safety and efficacy data for new products. For generic drugs, bioequivalence data or literature justification may be required.
For imported products, documents such as the Certificate of Pharmaceutical Product (CPP), Good Manufacturing Practice (GMP) certificate, and Letter of Authorization (LOA) from the manufacturer are mandatory. Additionally, product labeling must be in accordance with local language and regulatory requirements.
Once approved, a Marketing Authorization Certificate (MAC) is issued. The validity of the registration is typically 5 years, after which renewal is required. Companies must also comply with post-marketing obligations such as pharmacovigilance, adverse event reporting, and notification of any variations or changes to the product.


Registration Process
Portal Access & Login Credentials
To submit registration dossier and payment receipts, access to the DFDA’s official online portal, www.fdamyanmar.gov.mm, is required:
Login ID and password are mandatory and are issued only to authorized local representatives based in Myanmar. If you are a foreign manufacturer, your local partner or agent must hold valid credentials to access the portal.
Note:
Only an authorized local company in Myanmar may:
Submit documents
Apply for sample import permits
Communicate with DFDA on company's behalf
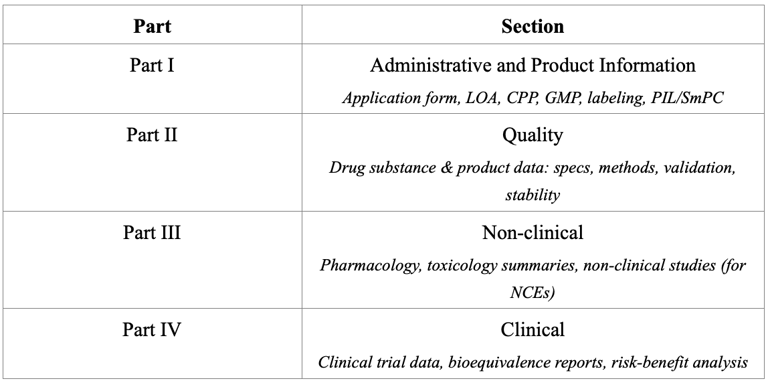
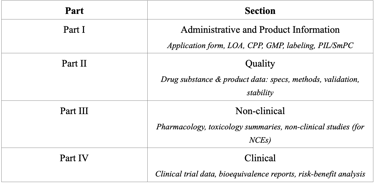
Registration Dossier Structure
Myanmar adopts the ASEAN Common Technical Dossier (ACTD) format for drug registration. The dossier consists of four parts, each covering a specific set of data.
Submission of Sample Permit application
Sample Permit
Import and submit drug samples to FDA
Submit Form 1 (Application form) + Registration Dossier + fees payemnt
Acknowledgement of complete dossier
Evaluation
Receipt of Preview
(After responding to query recived, if any)
Payment of product approved for registration
Registration Certificate (Form II) (Only issued to authorized local representative)